Introduction: Chimeric antigen receptor T-cell therapy (CAR-T) has revolutionized the treatment of non-Hodgkin lymphoma (NHL). Despite having an increased risk of NHL, patients with pre-existing autoimmune disease (AID) were excluded from pivotal CAR-T trials due to concerns that CAR-T may exacerbate AID. As a result, there are limited data on the safety and efficacy of CAR-T for NHL in patients with AID. Interestingly, there is also growing evidence that CAR-T can be therapeutically effective for AID, such as in the treatment of systemic lupus erythematosus (SLE). As CAR-T utilization for NHL increases, it is essential to characterize the safety and efficacy of this potentially curative therapy in patients with AID. This study aimed to evaluate the impact of AID on outcomes in patients treated with commercial CD19-targeted CAR-T for NHL.
Methods: Following Institutional Review Board approval, we conducted a retrospective analysis of all patients who were treated with commercial CD19-targeted CAR-T for NHL and had a pre-existing AID across the Mayo Clinic Enterprise (Arizona, Florida, and Minnesota) between January 2018 and May 2023. Variables collected included age, gender, NHL type, AID type, and CAR-T product. Efficacy outcomes, including response rates at 6 and 12 months after CAR-T, were collected. Toxicities, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), were collected and analyzed. Our chart review for AID included SLE, sarcoidosis, ulcerative colitis, celiac disease, pernicious anemia, Crohn's disease, psoriasis, Sjogren's syndrome, hypothyroidism (Hashimoto's disease), hyperthyroidism (Graves' disease), rheumatoid arthritis (RA), vasculitis, scleroderma, and mixed connective tissue disease (MCTD).
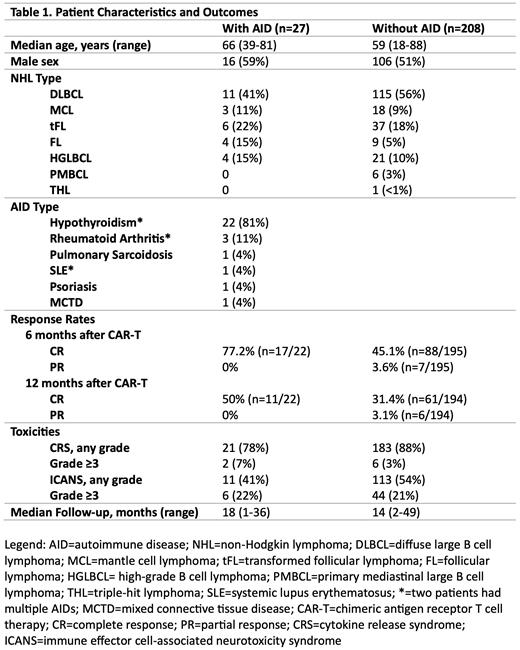
Results: A total of 235 patients who received commercial CD19-targeted CAR-T for NHL across the Mayo Clinic Enterprise were identified (Table 1). Of these 235 patients, 27 had a pre-existing AID and 208 did not. The median age was 66 years (range 39-81) for patients with AID and 59 years (range 18-88) for patients without AID. Over half of the patients were male (59% of patients with AID and 51% of patients without AID). The most common NHL type was diffuse large B cell lymphoma (41% of patients with AID and 56% of patients without AID). Most patients received axicabtagene ciloleucel (85% of patients with AID and 84% of patients without AID). The most common AID was hypothyroidism (81%, n=22), although other conditions were also identified, including RA (11%, n=3), SLE (4%, n=1), pulmonary sarcoidosis (4%, n=1), psoriasis (4%, n=1), and MCTD (4%, n=1). Two patients had more than one AID. Median follow-up was 18 months (range 1-36) for patients with AID and 14 months (range 2-49) for patients without AID.
Six months after CAR-T, the complete response (CR) rate was 77.2% and 45.1% in patients with AID and without AID, respectively (p=0.0042). Twelve months after CAR-T, the CR rate was 50% and 31.4% in patients with AID and without AID, respectively (p=0.0801). CRS of any grade occurred in 78% and 88% of patients with and without AID, respectively. ICANS of any grade occurred in 41% and 54% of patients with and without AID, respectively.
Conclusion: AID did not appear to adversely impact the safety or efficacy of CAR-T for NHL. Limitations of our study include the small number of patients with AID and the fact that the most common AID was hypothyroidism. Larger studies are warranted to confirm these findings. Additional data are needed to characterize the impact of CAR-T for NHL on AID outcomes and to describe the effect of disease-modifying antirheumatic drugs on NHL outcomes after CAR-T.
Disclosures
Gaulin:ADC Therapeutics: Consultancy; DeciBio: Consultancy. Wang:Genmab: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Novartis: Research Funding; Genentech: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Alhaj Moustafa:CSL Behring: Consultancy; Abbvie: Consultancy; Acrotech Biopharma: Research Funding. Murthy:CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Senti Biosciences: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bavarian Nordic: Membership on an entity's Board of Directors or advisory committees. Tsang:Poseida Therapeutics: Current holder of stock options in a privately-held company. Maurer:Roche/Genentech: Research Funding; BMS: Consultancy, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding. Paludo:AbbVie: Consultancy; Biofourmis: Research Funding; Karyopharm: Research Funding. Palmer:CTI BioPharma Corp.: Consultancy, Honoraria, Other: Money went to institution; Sierra Oncology: Consultancy, Other: Money went to Institution; morphosys: Consultancy, Other: Money went to institution; Jubliant: Consultancy; Incyte: Consultancy, Other: Money went to the institution. Villasboas:Regeneron: Research Funding; Aptose: Research Funding; Epizyme: Research Funding; Enterome: Research Funding; CRISPR: Research Funding; Genentech: Research Funding. Ansell:Affirmed: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research; ADC Therapeutics: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research. Nowakowski:ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Curis: Consultancy; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bantam Pharmaceutical LLC: Consultancy; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy; Blueprint Medicines: Consultancy; Celgene Corporation: Consultancy; Debiopharm: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Genentech: Consultancy; Incyte: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy; Kymera Therapeutics: Consultancy; Seagen: Consultancy; Selvita Inc: Consultancy; Abbvie: Consultancy; TG Therapeutics: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Zai Lab Limited: Consultancy. Munoz:Verastem: Consultancy, Speakers Bureau; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Acrotech/Aurobindo: Consultancy, Speakers Bureau; Physicians' Education Resource: Honoraria; Curio: Honoraria; OncView: Honoraria; Targeted Oncology: Honoraria; Millennium: Research Funding; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Incyte: Research Funding; Portola: Research Funding; Merck: Research Funding; Lilly/Loxo: Consultancy; TG Therapeutics: Consultancy; MEI: Consultancy; Morphosys/Incyte: Consultancy; Beigene: Consultancy, Research Funding, Speakers Bureau; Epizyme: Consultancy; ADC Therapeutics: Consultancy; Genmab: Consultancy; Karyopharm: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion: Consultancy; Kyowa: Honoraria, Speakers Bureau; Celgene/ Bristol-Myers Squibb: Consultancy, Speakers Bureau; Celgene: Research Funding; Pharmacyclics/ Janssen: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy; Bayer: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics/Abbvie: Consultancy, Research Funding; AstraZeneca: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal